Week 5:
Carver College of Medicine
June 23, 2014
This week was all about luciferase assays. The first assay was a NanoLuc luciferase assay (glow type) done on a 24-well plate and a 96-well plate. These wells contained the wild type neurons and the one with R235A mutant. We treated these neurons with 0, 12, 24, and 48 hours of rapamycin exposure and took a sample of each treatment at 12, 36, 48, and 60 hours. The neurons transfected with R235A mutant was expected to be resistant to rapamycin and thus served as the control. Therefore, the survival conditions should not be any different for different length of exposures to rapamycin for all transfected neurons. Wild type neurons, on the other hand, should show differences by measuring luciferase activities with the luminometer since they were not rapamycin-resistant.
The second assay was conducted with both Gaussia and NanoLuc luciferases on eight 24-well plates and two 96-well plates. Just as I described before, Gaussia luciferase was secreted by cells into the media and could tell us the relative number of cells in each well. This piece of data would be used for normalization purposes. NanoLuc luciferase, however, was contained in the cell body. After being lysed open by the lysis buffer, the amount of NanoLuc could tell us the survival condition of the cells.
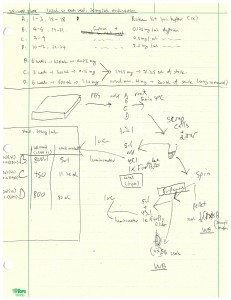
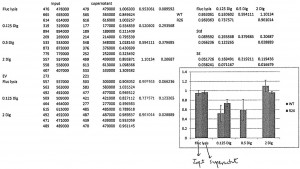
The last assay I did this week was a cell fractionation and Bβ2 luciferase reporter assay using Firefly luciferase, if we had to give it a name. The cells in the 24-well plate were either the wild type neurons or the ones containing R26 mutant. The customed lysis buffer was 50 mL and had 25 mM HEPES (pH = 7.5), 1 mM EDTA, 0.5 mM PMSF, and 100 mM NaCl. I made this buffer from our pre-made stock solutions of 500 mM EDTA (100 µL), 250 mM HEPES and 500 mM NaCl (5.00 mL), 4 M NaCl (625 µL), and 200 mM PMSF (125 µL). The 24-well plate was divided into groups A to D. Group A included wells 1 – 3 and 15 – 18 and was treated simply with 100 µL of Firefly lysis buffer. Group B contained wells 4 – 6 and 19 – 21 and was treated with 100 µL of 0.125 mg/mL of digitonin. Group C, which contained wells 7 – 9, was treated with 100 µL of 0.500 mg/mL digitonin. Group D had wells 10 – 12 and 22 – 24 and was treated with 100 µL of 2.00 mg/mL digitonin. Digitonin was a compound that was supposed to only break up the plasma cell membrane but not the mitochondrial membrane at the right concentration. By treating cells with different digitonin concentrations, we could identify the concentration that broke most of the cell membrane without lysing too much of the mitochondrial membrane apart. The experimental procedure was similar to that of last week. In short, we first lysed the cells open and saved a sample as the input that was measured by luminometer for luciferase activity. Then we spinned the cells down and added 1X sample buffer to the pellet which would be run on Western blot. We took a sample of the supernatant which was measured the same way for luciferase activity. We added 4X sample buffer to the rest of the supernatant which would also be run on Western blot. The flow chart of this assay was shown below. For this Bβ2 investigation, we expected the wild type to have luciferase inside mitochondria and the mutant to block the Bβ2 terminus of luciferase and prevent it from entering the mitochondrial membrane. Therefore, for wild type we expected the luciferase activity to be inside mitochondria (the pellet) and not in cytosol (supernatant). We would expect the exact opposite for the R26 mutants. The results of the luciferase activity assays were shown in the graph below. We could see that the 0.125 mg/mL digitonin concentration was not sufficient to lyse the cell membrane open completely. The 2.00 mg/mL concentration lysed all membranes open, which was the reason the graph showed no difference between the input and supernatant at that concentration. Therefore, another assay with 0.5 mg/mL, which was likely the concentration we were looking for, seemed imperative.
My birthday was also this week and the research scientist in our lab, Ron, made me a cake and I had a wonderful day. I also watched Ron running some samples for Western blot with 32P label, which was radioactive. The photos below showed me wearing the radioactive vest.


Major: Chemistry. Hometown:Centuria, Wisconsin.


